Explain why is ortho nitrophenol more acidic than ortho methoxyphenol?
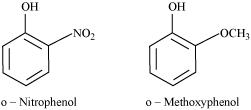
The nitro-group is an electron-withdrawing group. The presence of this group in the ortho position decreases the electron density in the O-H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
On the other hand, methoxy group is an electron-releasing group. Thus, it increases the electron density in the O-H bond and hence, the proton cannot be given out easily.
For this reason, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: