For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained.
| t (sec) | P(mm of Hg) |
| 0 | 35.0 |
| 360 | 54.0 |
| 720 | 63.0 |
Calculate the rate constant
The decomposition of azoisopropane to hexane and nitrogen at 543 K is represented by the following equation.
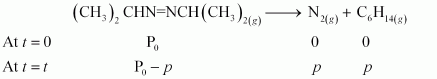
After time, t, total pressure, Pt = (Pº - p) + p + p
⇒ Pt = (Pº + p)
⇒ p = Pt - Pº
therefore, Pº - p = Pº - Pt - Pº
= 2 Pº - Pt
For a first order reaction,
k = 2.303/t Log Pº / Pº - p
= 2.303/t Log Pº / 2 Pº - Pt
When t = 360 s, k = 2.303 / 360s log 35.0 / 2x35.0 - 54.0
= 2.175 × 10 - 3 s - 1
When t = 720 s, k = 2.303 / 720s log 35.0 / 2x35.0 - 63.0
= 2.235 × 10 - 3 s - 1
Hence, the average value of rate constant is
k = (2.175 × 10 - 3 + 2.235 × 10 - 3 ) / 2 s - 1
= 2.21 × 10 - 3 s - 1
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: