Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3-
(iv) [CoF6]3-
(i) [Fe(CN)6]4-
In the above coordination complex, iron exists in the +II oxidation state.
Fe2+ : Electronic configuration is 3d6
Orbitals of Fe2+ ion:

Hence, the geometry of the complex is octahedral and the complex is diamagnetic (as there are no unpaired electrons).
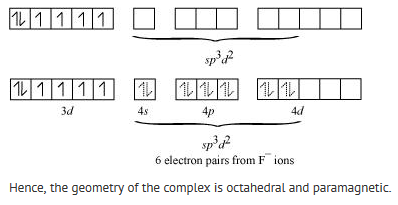
(ii) [FeF6]3-
In this complex, the oxidation state of Fe is +3.
Orbitals of Fe+3 ion:

Hence, the geometry of the complex is found to be octahedral.
(iii) [Co(C2O4)3]3-
Cobalt exists in the +3 oxidation state in the given complex.
Orbitals of Co3+ ion:

Hence, the geometry of the complex is found to be octahedral.
(iv) [CoF6]3- Cobalt exists in the +3 oxidation state.
Orbitals of Co3+ ion:

Again, fluoride ion is a weak field ligand. It cannot cause the pairing of the 3d electrons. As a result, the Co3+ ion will undergo sp3d2 hybridization. sp3d2 hybridized orbitals of Co3+ ion are:
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: