The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
In an aqueous solution, KOH almost completely ionizes to give OH - ions. OH - ion is a strong nucleophile, which leads the alkyl chloride to undergo a substitution reaction to form alcohol.
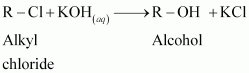
On the other hand, an alcoholic solution of KOH contains alkoxide (RO - ) ion, which is a strong base. Thus, it can abstract a hydrogen from the β-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.

OH - ion is a much weaker base than RO - ion. Also, OH - ion is highly solvated in an aqueous solution and as a result, the basic character of OH - ion decreases. Therefore, it cannot abstract a hydrogen from the β-carbon.
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: