Complete each synthesis by giving missing starting material, reagent or products
(i)
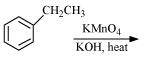
(ii)

(iii)

(iv)

(v)

(vi)
(i)

(ii)

(iii)

(iv)

(v)

(vi)

(vii)

(viii)

(ix)

(x)

(xi)

The rate constant for the decomposition of N2O5 at various temperatures is given below:
|
T/°C |
0 | 20 | 40 | 60 | 80 |
|
105 X K /S-1 |
0.0787 | 1.70 | 25.7 | 178 | 2140 |
Draw a graph between ln k and 1/T and calculate the values of A and Ea.
Predict the rate constant at 30 º and 50 ºC.
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: