Why does O3 act as a powerful oxidising agent?
Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free radical, is very reactive.
O3 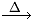 O2 + [O]
O2 + [O]
Ozone Oxygen Nascent Oxygen
Therefore, ozone acts as a powerful oxidising agent.
NCERT questions are designed to test your understanding of the concepts and theories discussed in the chapter. Here are some tips to help you answer NCERT questions effectively:
Welcome to the NCERT Solutions for Class 12 Chemistry - Chapter . This page offers a step-by-step solution to the specific question from Excercise 1 , Question 18: Why does O3 act as a powerful oxidising agent?....
Comments
This site is a great site. A lot of thanks
yes
Thanks