The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
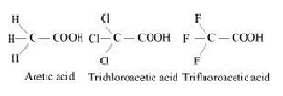
The above trend in the depression in the freezing point of water for the same amount of given compounds can be explained on the basis of degree of ionization, which depends upon the strength of the acid.Trifluroacetic acid is more acidic than trichloroacetic acid which is further more acidic than acetic acid.
Therefore the degree of ionization of these acids will decrease in the following order:
Trifluoroactic acid > trichloroacetic acid > acetic acid
Now greater the degree of ionization ,greater will be the depression of freezing point.
NCERT questions are designed to test your understanding of the concepts and theories discussed in the chapter. Here are some tips to help you answer NCERT questions effectively:
Welcome to the NCERT Solutions for Class 12 Chemistry - Chapter . This page offers a step-by-step solution to the specific question from Excercise 2 , Question 31: The depression in freezing point of water observed for the same amount of acetic acid, trichloroacet....
Comments