The rate constant for the decomposition of N2O5 at various temperatures is given below:
|
T/°C |
0 | 20 | 40 | 60 | 80 |
|
105 X K /S-1 |
0.0787 | 1.70 | 25.7 | 178 | 2140 |
Draw a graph between ln k and 1/T and calculate the values of A and Ea.
Predict the rate constant at 30 º and 50 ºC.
From the given data, we obtain
| T/°C | 0 | 20 | 40 | 60 | 80 |
|
T/K |
273 | 293 | 313 | 333 | 353 |
| 1/T / k-1 |
3.66×10 - 3 |
3.41×10 - 3 |
3.19×10 - 3 |
3.0×10 - 3 |
2.83 ×10 - 3 |
| 105 X K /S-1 | 0.0787 | 1.70 | 25.7 | 178 | 2140 |
| In k | -7.147 | -4.075 | -1.359 | -0.577 | 3.063 |
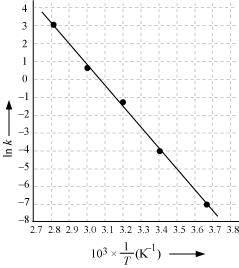
Slope of the line,

In k= - 2.8
Therefore, k = 6.08x10-2s-1
Again when T = 50 + 273K = 323K,
1/T = 3.1 x 10-3 K
In k = - 0.5
Therefore, k = 0.607 s-1
NCERT questions are designed to test your understanding of the concepts and theories discussed in the chapter. Here are some tips to help you answer NCERT questions effectively:
Welcome to the NCERT Solutions for Class 12 Chemistry - Chapter . This page offers a step-by-step solution to the specific question from Excercise 2 , Question 22: The rate constant for the decomposition of N2O5 at various temperatures is given below: ....
Comments
Gud