The time required for 10% completion of a first order reaction at 298 K is equal to that required for its 25% completion at 308 K. If the value of A is 4 x 1010 s-1. Calculate k at 318 K and Ea.
For a first order reaction,
t = 2.303 / k log a / a - x
At 298 K,
t = 2.303 / k log 100 / 90
= 0.1054 / k
At 308 K,
t' = 2.303 / k' log 100 / 75
= 2.2877 / k'
According to the question,
t = t'
⇒ 0.1054 / k = 2.2877 / k'
⇒ k' / k = 2.7296
From Arrhenius equation,we obtain
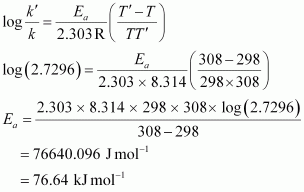
To calculate k at 318 K,
It is given that, A = 4 x 1010 s-1, T = 318K
Again, from Arrhenius equation, we obtain

Therefore, k = Antilog (-1.9855)
= 1.034 x 10-2 s -1
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: