The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
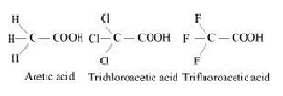
The above trend in the depression in the freezing point of water for the same amount of given compounds can be explained on the basis of degree of ionization, which depends upon the strength of the acid.Trifluroacetic acid is more acidic than trichloroacetic acid which is further more acidic than acetic acid.
Therefore the degree of ionization of these acids will decrease in the following order:
Trifluoroactic acid > trichloroacetic acid > acetic acid
Now greater the degree of ionization ,greater will be the depression of freezing point.
The rate constant for the decomposition of N2O5 at various temperatures is given below:
|
T/°C |
0 | 20 | 40 | 60 | 80 |
|
105 X K /S-1 |
0.0787 | 1.70 | 25.7 | 178 | 2140 |
Draw a graph between ln k and 1/T and calculate the values of A and Ea.
Predict the rate constant at 30 º and 50 ºC.
Comments
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications:
Taking Screenshots on your Samsung Galaxy M31s is very easy and quick.
Report a problem on Specifications: