Complete NCERT Solutions Guide
Access step-by-step solutions for all NCERT textbook questions
Welcome to the Chapter 2 - Solutions, Class 12 Chemistry NCERT Solutions page. Here, we provide detailed question answers for Chapter 2 - Solutions. The page is designed to help students gain a thorough understanding of the concepts related to natural resources, their classification, and sustainable development.
Our solutions explain each answer in a simple and comprehensive way, making it easier for students to grasp key topics Solutions and excel in their exams. By going through these Solutions question answers, you can strengthen your foundation and improve your performance in Class 12 Chemistry. Whether you’re revising or preparing for tests, this chapter-wise guide will serve as an invaluable resource.
A solution is a homogeneous mixtures of two or more than two substances on molecular level.The constitutent of the mixture present in a smaller amount is called the SOLUTE & the one present in larger amount is called the SOLVENT.For eg. Small amount of sugar(solute) dissolved in water(solvent).
SOLUTE + SOLVENT = SOLUTION

There are nine types of solutions formed.They are:
|
Sno |
State of solute |
State of solvent |
Examples |
|
1 |
GAS |
GAS |
Air |
|
2 |
GAS |
LIQUID |
Oxygen in water |
|
3 |
GAS |
SOLID |
Smole particles in air |
|
4 |
LIQUID |
GAS |
Carbon dioxide dissolved in water |
|
5 |
LIQUID |
LIQUID |
Alcohol in water |
|
6 |
LIQUID |
SOLID |
Mercury in silver |
|
7 |
SOLID |
GAS |
Adsorption of hydrogen over palladium |
|
8 |
SOLID |
LIQUID |
Sugar in water |
|
9 |
SOLID |
SOLID |
Carbon in Iron(steel) |
Out of these nine types of solution , solid in liquid, liquid in liquid & gas in liquid are very common.When the components of the solution are mixed,the resulting solution may be in the solid, liquid or gaseous state.They are
(i) Gaseous solution:The solution in which the solvent is a gas is called a gaseous solution. In these solutions, the solute may be liquid, solid, or gas. For example, a mixture of oxygen and nitrogen gas is a gaseous solution.
(ii) Liquid solution:The solution in which the solvent is a liquid is known as a liquid solution. The solute in these solutions may be gas, liquid, or solid.
(iii) Solid solutions: The solution in which the solvent is a solid is known as a solid solution.The solute in these solutions may be a gas, liquid or solid. For example, a solution of copper in gold is a solid solution.
The lower members of alcohols are highly soluble in water but the solubility decreases with increase in the molecular weight. The solubility of lower alcohols in water is due to formation of hydrogen bonds(Hydrogen bonding) between alcohols & water molecules.

However, as the size of alcohol molecule increases, the alkyl groups becomes larger & prevents the formation of hydrogen bonds with water, & hence the solubility goes on decreasing with increase in the length of carbon chain.Also the interaction between the molecules of alcohol and water is weaker than alcohol−alcohol and water−water interactions. As a result, when alcohol and water are mixed, the intermolecular interactions become weaker and the molecules can easily escape. This increases the vapour pressure of the solution, which in turn lowers the boiling point of the resulting solution.
The dissolution of a gas in a liquid is exothermic process.Therefore according to LeChatelier principle,with the increase in temperature,the equilibrium shifts in the backward direction.
Gas + Liquid → Solution + Heat
Therefore the solubility of gas in solution decreases with rise in temperature.
Henry’s law states that the mass of a gas dissolved per unit volume of the solvent at a given temperature is proportional to the pressure of the gas in equilibrium with the solution.
Or
It also states that the pressure of a gas over a solution in which the gas is dissolved is proportional to the mole fraction of the gas dissolved in the solution.
The important applications of Henry’s law are as follows:
1) In the production of carbonated beverages-in order to increase the solubility of CO2 in cold drinks,beer etc., the bottle are sealed under high pressure. When the bottle is opened under normal atmospheric pressure, the pressure inside the bottle falls to atmospheric pressure & the excess CO2 bubbles out of the bottle causing effervescence.
2) At high altitudes-the partial pressure of oxygen at high altitudes is less than the ground level. This results in low concentration of oxygen in the blood & tissues of the peoples.
3) In scuba diving- during scuba diving,when the diver breaths in compressed air from the supply tank, more nitrogen dissolves in the blood & other body fluids because the pressure at that depth is far greater than the surface atmospheric pressure.
According to Henry’s law
.m = k x p
Substituting the given values in the above equation.
We get
6.56 x10-3 = k x 1
Or
k = 6.56 x10-3
Now when m = 5 x 10-2,
Then again substituting the given values in Henry’s law equation, we get
5 x 10-2 = 6.56 x10-3 x p
Or
p = 7.62 bar
Raoult’s law states that at a given temperature, the vapour pressure of a solution containing non volatile solute is directly proportional the mole fraction of the solvent.
Non ideal solutions shows positive & negative deviations from ideal behavoiur.
Non ideal solutions showing positive deviations from Raoult’s law- Consider a binary solution of two components A & B .If the A-B interaction in the solutions are weaker than A-A & B-B interactions in the two liquids forming the solution,then the escaping tendency of A & B types of molecules from the solution becomes more than from pure liquids.
As a result ,each component of solution has a partial vapour pressure greater than expected on the basis of Raoult’s law.This is called positive deviations from Raoult’s law,i.e PA> PA °xA & PB >PB°xB
ΔmixH is positive because energy is required to break A-A & B-B attractive forces.Hence endothermic process.
Non ideal solutions showing Negative deviations from Raoult’s law- in such solutions,the A-B interactions are stronger than A-A & B-B interactions .Due to stronger A-B interactions ,the escaping tendency of A & B types of molecules from the solution becomes less than from pure liquids. Consequently, each component of the solution has a partial vapour pressure less than expected on the basis of Raoults law. This is called negative deviations form Raoults law,i.e PA< PA °xA & PB B°xB
ΔmixH is negative because energy is released due to increase in attractive forces. Hence exothermic process.
Here,
Vapour pressure of the solution at normal boiling point (p1) = 1.004 bar (Given)
Vapour pressure of pure water at normal boiling point (p10) = 1.013 bar
Mass of solute, (w2) = 2 g
Mass of solvent (water), (w1) = 100 - 2 = 98 g
Molar mass of solvent (water), (M1) = 18 g mol - 1
According to Raoult's law,
(p10 - p1) / p10 = (w2 x M1 ) / (M2 x w1 )
(1.013 - 1.004) / 1.013 = (2 x 18) / (M2 x 98 )
0.009 / 1.013 = (2 x 18) / (M2 x 98 )
M2 = (2 x 18 x 1.013) / (0.009 x 98)
M2 = 41.35 g mol - 1
Hence, the molar mass of the solute is 41.35 g mol - 1.
Vapour pressure of heptane p10 = 105.2 kPa
Vapour pressure of octane p20= 46.8 kPa
As we know that, Molar mass of heptane (C7H16) = 7 × 12 + 16 × 1 = 100 g mol - 1
∴ Number of moles of heptane = 26/100 mol = 0.26 mol
Molar mass of octane (C8H18) = 8 × 12 + 18 × 1 = 114 g mol - 1
∴ Number of moles of octane = 35/114 mol = 0.31 mol
Mole fraction of heptane, x1 = 0.26 / 0.26 + 0.31
= 0.456
And, mole fraction of octane, x2 = 1 - 0.456 = 0.544
Now, partial pressure of heptane, p1 = x2 p20
= 0.456 × 105.2
= 47.97 kPa
Partial pressure of octane,p2 = x2 p20
= 0.544 × 46.8 = 25.46 kPa
Hence, vapour pressure of solution, ptotal = p1 + p2
= 47.97 + 25.46
= 73.43 kPa
1 molal solution means 1 mol of the solute is present in 1000 g of the solvent (water).
Molar mass of water = 18 g mol - 1
∴ Number of moles present in 1000 g of water = 1000/18
= 55.56 mol
Therefore, mole fraction of the solute in the solution is
x2 = 1 / (1+55.56) = 0.0177.
It is given that,
Vapour pressure of water, p10 = 12.3 kPa
Applying the relation, (P10 - P1) / P10 = X2
⇒ (12.3 - p1) / 12.3 =0.0177
⇒ 12.3 - P1 = 0.2177
⇒ p1 = 12.0823
= 12.08 kPa (approximately)
Hence, the vapour pressure of the solution is 12.08 kPa.
Let the vapour pressure of pure octane be p10.
Then, the vapour pressure of the octane after dissolving the non-volatile solute is 80/100 p10 = 0.8 p10.
Molar mass of solute, M2 = 40 g mol - 1
Mass of octane, w1 = 114 g
Molar mass of octane, (C8H18), M1 = 8 × 12 + 18 × 1 = 114 g mol - 1
Applying the relation,
(p10 - p1) / p10 = (w2 x M1 ) / (M2 x w1 )
⇒ (p10 - 0.8 p10) / p10 = (w2 x 114 ) / (40 x 114 )
⇒ 0.2 p10 / p10 = w2 / 40
⇒ 0.2 = w2 / 40
⇒ w2 = 8 g
Hence, the required mass of the solute is 8 g.
Let, the molar mass of the solute be M g mol - 1
Now, the no. of moles of solvent (water),n1 = 90g / 18g mol-1
And, the no. of moles of solute,n2 = 30g / M mol-1 = 30 / M mol
p1 = 2.8 kPa
Applying the relation:
(p10 - p1) / p10 = n2 / (n1 + n2)
⇒ (p10 - 2.8) / p10 = (30/M) / {5 + (30/M)}
⇒ 1 - (2.8/p10) = (30/M) / {(5M+30)/M}
⇒ 1 - (2.8/p10) = 30 / (5M + 30)
⇒ 2.8/p10 = 1 - 30 / (5M + 30)
⇒ 2.8/p10 = (5M + 30 - 30) / (5M + 30)
⇒ 2.8/p10 = 5M / (5M+30)
⇒ p10 / 2.8 = (5M+30) / 5M ----------------(1)
After the addition of 18 g of water:
n1 = (90+18g) / 18 = 6 mol
and the new vapour pressure is p1 = 2.9 kPa (Given)
Again, applying the relation:
(p10 - p1) / p10 = n2 / (n1 + n2)
⇒ (p10 - 2.9) / p10 = (30/M) / {6 + (30/M)}
⇒ 1 - (2.9/p10) = (30/M) / {(6M+30)/M}
⇒ 1 - (2.9/p10) = 30 / (6M + 30)
⇒ 2.9/p10 = 1 - 30 / (6M + 30)
⇒ 2.9/p10 = (6M + 30 - 30) / (6M + 30)
⇒ 2.9/p10 = 6M / (6M+30)
⇒ p10 / 2.9 = (6M+30) / 6M ----------------(2)
Dividing equation (1) by (2),we get:
2.9 / 2.8 = {(5M+30) / 5M} / {(6M+30) / 6M}
⇒ 2.9 x (6M+30 / 6) = (5M+30 / 5) x 2.8
⇒ 2.9 x (6M +30) x 5 = (5M+30) x 2.8 x 6
⇒ 87M + 435 = 84M + 504
⇒ 3M = 69
⇒ M = 23u
Therefore, the molar mass of the solute is 23 g mol - 1.
(ii) Putting the value of 'M' in equation (i), we get:
⇒ p10 / 2.8 = (5M+30) / 5M
⇒ p10 / 2.8 = (5x23+30) / 5x23
⇒ p10 = (145 x 2.8) / 115
⇒ p10 = 3.53
Hence, the vapour pressure of water at 298 K is 3.53 kPa.
The given solid solution is the example of interstitial solid solution.
For example: tungsten carbide, where tungsten atoms are arranged in a face centred cubic pattern with carbon atoms in octahedral holes.
In case of cane sugar:
ΔTf = (273.15 - 271) K = 2.15 K
Molar mass of sugar (C12H22O11) = 12 × 12 + 22 × 1 + 11 × 16 = 342 g mol - 1
5% solution (by mass) of cane sugar in water means 5 g of cane sugar is present in (100 - 5)g = 95 g of water.
Now, number of moles of cane sugar =5/342 mol
= 0.0146 mol
Therefore, molality of the solution,m =0.0146mol / 0.095kg
= 0.1537 kg mol - 1
Now applying the relation,
ΔTf = Kf × m
⇒ Kf = ΔTf / m
⇒ 2.15K / 0.1537 kg mol-1
= 13.99 K kg mol-1
Molar of glucose (C6H12O6) = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol - 1
5% glucose in water means 5 g of glucose is present in (100 - 5) g = 95 g of water.
∴ Number of moles of glucose = 5/180 mol
= 0.0278 mol
Therefore, molality of the solution, m =0.0278 mol / 0.095 kg
= 0.2926 mol kg - 1
Applying the relation,
ΔTf = Kf × m
= 13.99 K kg mol - 1 × 0.2926 mol kg - 1
= 4.09 K (approximately)
Hence, the freezing point of 5% glucose solution is (273.15 - 4.09) K= 269.06 K.
As We know that:
MB = (Kf x wB x 1000) / (wA X ΔTf)
Now ΔTf = 2.3 , wB = 1.0 , wA = 20, KF = 5.1 (given)
PUTTING THE VALUES IN THE EQUATION
MB = (5.1 x 1 x 1000) / (20 x 2.3) = 110.87 g/mol
Therefore MAB2 = 110.9
For AB4 compound
ΔTf = 1.3 , wb = 1 ,wa = 20
MB = (5.1 X 1 X 1000) / (20 X 1.3) = 196 g/mol
Therefore MAB = 196
Let x be the atomic mass of A & y be the atomic mass of B,
THEN MAB2 = x + 2y = 110.9 ----------------------------------(1)
And MAB = x + 4y = 196 ----------------------------------(2)
Subtracting 2 from 1 ,we get
2y = 196-110.9
y = 85.1 / 2
y = 42.6
Putting the value of y in 1 we get
x = 110.9 - 2 x 42.6
x = 25.7
Therefore atomic mass of A = 25.7 u Atomic mass of B = 42.6 u.
Here we have given
π1= 4.98
π2 = 1.52
C1 = 36/180
C2 = ? (we have to find)
Now according to van’t hoff equation
Π = CRT
Putting the values in above equation,we get
4.98 = 36/180RT ------------------------1
1.52 = c2RT ------------------------2
Now dividing equation 2 by 1 ,we get
(c2 x 180) / 36 = 1.52 / 4.98
or
c2 = 0.061
Therefore concentration of 2nd solution is 0.061 M
1) both of them has van der waals interactions
2) both of them has van der waals interactions
3) both of them has ion dipole interactions
4) both of them has hydrogen bonding
5) both of them has dipole-dipole interactions
n-octane is an organic solvent (liquid)
Out of given examples cyclohexane is strongest organic solvent, so according to like dissolves like, cyclohexane is most likely to be dissolved as a solute in n octane. After cyclohexane, CH3CN
Will be dissolved completely as a solute in n octane, then CH3OH & the least soluble will be Kcl. Also since n-octane & cyclohexane, both of them belongs to alkane category, their solubility will be maximum.
Therefore the order of increasing solubility in n octane is as follows:
Kcl < CH3OH < CH3CN < Cyclohexane
(i) Phenol (C6H5OH) has the polar group -OH and non-polar group -C6H5. Thus, phenol is partially soluble in water.
(ii) Toluene (C6H5-CH3) has no polar groups. Thus, toluene is insoluble in water.
(iii) Formic acid (HCOOH) has the polar group -OH and can form H-bond with water. Thus, formic acid is highly soluble in water.
(iv) Ethylene glycol  has polar -OH group and can form H-bond. Thus, it is highly soluble in water.
has polar -OH group and can form H-bond. Thus, it is highly soluble in water.
(v) Chloroform is insoluble in water.
(vi) Pentanol (C5H11OH) has polar -OH group, but it also contains a very bulky non-polar -C5H11 group. Thus, pentanol is partially soluble in water.
We know molality = moles of solute / mass of solvent in kg
Now mass of Na+ ions = 92 g (given)
Moles of Na+ ions = 92 / 23 = 4
And mass of water = 1kg
Therefore molality = 4/1 = 4m
Solubility product of CuS, Ksp of CuS = 6 x 10-16
If s is the solubility,
then CuS = cu2+ + S2-
Therefore KSP = { cu2+}{ S2-}
Or
KSP = s x s
Or
s = √ KSP = √6 x 10-16
= 2.45 x 10-8 M
Mass of aspirin (C9H8O4) = 6.5g (given)
Mass of acetonitrile (CH3CN) = 450g (given)
Now total mass of the solution = 6.5 + 450 = 456.5g
Therefore mass percentage of aspirin (C9H8O4) = (6.5 / 456.5 ) x 100
= 1.42 %
Molecular mass of nalorphene (C19H21NO3),
= 19 x 12 + 21 x 1 + 1 x 14 + 3 x 16 = 311 g/mol
Moles of nalorphene (C19H21NO3) = 1.5 x 10-3 x 311 = 4.67 x 10-6 mol
Now molality = (moles of solute / mass of solvent in g) x 1000
Putting the values in above equation,we get
1.5 x 10-3 = (4.67 x 10-6 / mass of water) x 1000
Or
Mass of water = (4.67 x 10-6 x 1000) / 1.5 x 10-3
Therefore mass of water = 3.1g
a) Mole Fraction - it is defined as the ratio of number of moles of one component to the total number of moles of solute & solvent present in the solution.
Mole fraction = number of moles of the component / total number of moles of all components
It is denoted by x.
Take it as binary solution, the number of the moles of solute will be nA and the number of moles of solvent will be nB, then the mole fraction of the solute in the solution is given as:

In the same manner, the mole fraction of the solvent in the solution is given as:

b) Molality- it is the number of moles of the solute dissolved per 1000g of the solvent.
It is denoted by m
Molality = mole of solute / mass of solvent in kg
Unit for molality are moles/kg
c) Molarity- it is the number of moles of the solute dissolved per litre of the solution.
It is represented by M.
Molarity = moles of solute / volume of solute in litres
Unit for molarity is moles/litre
d) Mass Percentage- the mass percentage of a component in a given solution is the mass of the component per 100g of the solution.
Mass percentage of the component = (mass of the component in the solution/total mass of the solution) x100
We know Molarity = moles of the solute / volume of solution
Putting the given values in above equation,we get
0.15 = (mole of benzoic acid / 250) x 1000
Or
moles of benzoic acid = (0.15 x 250) / 1000
= 0.0375 mol of benzoic acid
Also molecular mass of benzoic acid (C6H5COOH)
= 7 × 12 + 6 × 1 + 2 × 16
= 122 g/mol
Therefore amount of benzoic acid = 0.0375 x 122 = 4.575 g
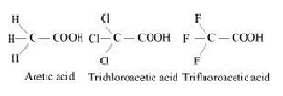
The above trend in the depression in the freezing point of water for the same amount of given compounds can be explained on the basis of degree of ionization, which depends upon the strength of the acid.Trifluroacetic acid is more acidic than trichloroacetic acid which is further more acidic than acetic acid.
Therefore the degree of ionization of these acids will decrease in the following order:
Trifluoroactic acid > trichloroacetic acid > acetic acid
Now greater the degree of ionization ,greater will be the depression of freezing point.
Molar mass of CH3CH2CHClCOOH
15 + 14 + 13 + 35.5 + 12 + 16 + 16 + 1
= 122.5 g/mol
∴ Moles of CH3CH2CHClCOOH = 10g / 122.5 g/mol
= 0.0816 mol
Therefore molality of the solution
= (0.0816 x 1000) / 250
= 0.3265 mol kg-1
Now if a is the degree of dissociation of CH3CH2CHClCOOH,

So, Ka = (Cα x Cα) / (C (1-α))
Ka = Cα2 / (1-α)
Since α is very small with respect to 1, 1 - α = 1
Ka = Cα2
α = √Kα / C
Putting the values ,We get
α = √1.4 x 10-3 / 0.3265
= 0.0655
Now at equilibrium,the van’t hoff factor i = 1-α +α +α/1
= 1 + 0.0655
= 1.0655
Hence, the depression in the freezing point of water is given as:
Therefore ΔTf = i Kf m v
= 1.065 v x 1.86 x 0.3265
= 0.647°
Molecular mass of CH2FCOOH
14 + 19 + 12 + 16 + 16 + 1 = 78 g/mol
Now, Moles of CH2FCOOH = 19.5 / 78
= 0.25
Taking the volume of the solution as 500 mL, we have the concentration:
C = (0.25 / 500) X 1000
Therefore Molality = 0.50m
So now putting the value in the formula :
ΔTf = Kf x m
=1.86 x 0.50 = 0.93K
Van’t hoff factor = observed freezing point depression / calculated freezing point depression
= 1 / 0.93 = 1.0753
Let α be the degree of dissociation of CH2FCOOH

Now total number of moles = m(1-a) + ma +ma = m(1+a)
Or
i = α(1+α) / α = 1 +α = 1.0753
Therefore α = 1.0753- 1
= 0.0753
Now the Value of Ka is given as:
Ka = [CH2FCOO-][H+] / CH2FCOOH
= (Cα x Cα) / (C (1-α))
= Cα2 / (1-α)
Ka = 0.5 X (0.0753)2 / (1-0.0753)
= 0.5 X 0.00567 / 0.09247
= 0.00307 (approx.)
= 3 X 10-3
Vapour pressure of water, p1° = 17.535 mm of Hg
Mass of glucose, w2 = 25 g
Mass of water, w1 = 450 g
We know that,
Molar mass of glucose (C6H12O6),
M2 = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol - 1
Molar mass of water, M1 = 18 g mol - 1
Then, number of moles of glucose, n1 = 25/180 = 0.139 mol
And, number of moles of water, n2 =450/18 = 25 mol
Now, we know that,
(p1° - p°) / p1° = n1 / n2 + n1
⇒ 17.535 - p° / 17.535 = 0.139 / (0.139+25)
⇒ 17.535 - p1 = 0.097
⇒ p1 = 17.44 mm of Hg
Hence, the vapour pressure of water is 17.44 mm of Hg.
As we know that
p = k x c
We have given p = 760 mm, k = 4.27 x 105
Putting the given values in equation
760 = 4.27 x 105 x c
Or
c = 760 / 4.27x 105
c = 178 x 10-5
Number of Moles of Liquid A, nA = 100 / 140 = 0.714
Number of Moles of Liquid B, nB = 1000 / 180 = 5.556
Then Mole fraction of A = nA / nA + nB = 0.714 / 0.714 + 5.556
= 0.114
Now Mole of fraction of B = 1 - 0.114 = 0.886
Now ptotal = pA + pB
Or ptotal = p°AXA + p°BxB
OR
475 = p°A X 0.114 + 500 X 0.886
OR
p°A = 280.7 torr
Therefore vapour pressure of pure A = 280.7 torr
Vapour pressure of A in solution = 280.7 x 0.114
= 32 torr
Now
pA = p°AXA
Or
p°A = pA / XA
⇒ 32 / 0.114
= 280.7 torr
Hence, the vapour pressure of pure liquid A is 280.7 torr.
Vapour pressure of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot ptotal' pchloroform' and pacetoneas a function of xacetone. The experimental data observed for different compositions of mixture is.
|
100 ×xacetone |
0 | 11.8 | 23.4 | 36.0 | 50.8 | 58.2 | 64.5 | 72.1 |
|
pacetone /mm Hg |
0 | 54.9 | 110.1 | 202.4 | 322.7 | 405.9 | 454.1 | 521.1 |
|
pchloroform/mm Hg |
632.8 | 548.1 | 469.4 | 359.7 | 257.7 | 193.6 | 161.2 | 120.7 |
From the question, we have the following data
|
100 ×xacetone |
0 | 11.8 | 23.4 | 36.0 | 50.8 | 58.2 | 64.5 | 72.1 |
|
pacetone /mm Hg |
0 | 54.9 | 110.1 | 202.4 | 322.7 | 405.9 | 454.1 | 521.1 |
|
pchloroform/mm Hg |
632.8 | 548.1 | 469.4 | 359.7 | 257.7 | 193.6 | 161.2 | 120.7 |
|
ptotal(mm Hg) |
632.8 | 603.0 | 579.5 | 562.1 | 580.4 | 599.5 | 615.3 | 641.8 |

It can be observed from the graph that the plot for the ptotalof the solution curves downwards. Therefore, the solution shows negative deviation from the ideal behaviour.
Molar mass of benzene(C6H6) = 6 X 12 + 6 X 1 = 78 g/mol
Molar mass of toluene = 7 x 12 + 8 x 1 = 92 g/mol
Now no of moles in 80g of benezen = 80 / 78 = 1.026 mol
No of moles in 100g of toluene = 100 / 92 = 1.087 mol
∴Mole fraction of benzene xb = 1.026 / 1.026 + 1.087 = 0.486
And Mole fraction of toluene,xt = 1 - 0.486 = 0.514
We have given that
Vapor pressure of pure benzene pb° = 50.71 mm Hg
And, vapour pressure of pure toluene, pt° = 32.06 mm Hg
Therefore partial Vapor pressure of benzene, pb = pb X xb
= 50.71 x 0.486
= 24.65 mm Hg
And partial Vapor pressure of toluene, pt = pt X xt
Pt = p°t X xt = 32.06 x 0.514
= 16.48
Total vapour pressure = 24.65 + 16.48 = 41.13 mm Hg
Mole fraction of benzene in vapour phase = 24.65 / 41.13 = 0.60
Percentage of oxygen (O2) in air = 20 %
Percentage of nitrogen (N2) in air = 79%
Also, it is given that water is in equilibrium with air at a total pressure of 10 atm, that is, (10 × 760) mm Hg = 7600 mm Hg
Therefore, Partial pressure of oxygen, po2 = 20/100 *7600
=1520 mm Hg
Partial pressure of nitrogen,pN2 = 79/100 *7600
= 6004 mmHg
Now, according to Henry's law:
p = KH.x
For oxygen:
po2 = KH. xO2
⇒xO2 = po2 / KH
= 1520 / 3.30 X 107
= 4.61 10-5
For nitrogen:
pN2 = KH.xN2
⇒xN2 = pN2 / KH
= 6004 / 6.51 x 107
= 9.22 x 10-5
Hence, the mole fractions of oxygen and nitrogen in water are 4.61 ×10 - 5 and 9.22 × 10 - 5 respectively.
Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in an aqueous solution. This means that 68 g of nitric acid is dissolved in 100 g of the solution.
Molar mass of nitric acid (HNO3) = 1 × 1 + 1 × 14 + 3 × 16 = 63 g mol - 1
Then, number of moles of HNO3 = 68 / 63 mol
= 1.08 mol
Also density = 1.504g/mL-1 (given)
Therefore from the formula density = mass / volume, we get
Volume of solution = 1000/1.504 = 66.49 mL
Therefore molarity of nitric acid = (1.08/66.49) x 1000 = 16.24 M
We know that
π = i n/V RT
⇒π = i w/MV iRT
⇒ w = πMV / iRT .......................(1)
Now we have given below values:
π = 0.75 atm
V = 2.5L
i = 2.47
T = (27+273) K = 300K
Here,
R = 0.0821L atm k-1 mol-1
M = 1x40 + 2x35.5
= 111 g/mol
Now putting the value in equation 1:
w = 0.75x111x2.5 / 2.47x0.0821x300
=3.42g
Hence, the required amount of CaCl2 is 3.42 g.
If K2SO4 is completely dissociated then these ions are produced
Then K2SO4 = 2K+ + SO42-
So total number of ions produced = 3
Therefore I =3
Now molecular mass of K2SO4 = 2 x 39 + 1 x 32 + 4 x 16 = 174 g/mol
Now π = I Crt
= I WB X RT / MB X V
= 3 X 25 X 10-3 X 0.082 X 298 / 174 X 2
= 5.27 X 10-3 atm.
10% w/w solution of glucose in water means that 10 g of glucose in present in 100 g of the solution i.e., 10 g of glucose is present in (100 - 10) g = 90 g of water.
Molar mass of glucose (C6H12O6) = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol - 1
Then, number of moles of glucose = 10 / 180 mol
= 0.056 mol
∴ Molality of solution = 0.056 mol / 0.09kg = 0.62 m
Number of moles of water = 90g / 18g mol-1 = 5 mol
Mole fraction of glucose (xg) = 0.056 / ( 0.056+5) = 0.011
And, mole fraction of water xw = 1 - xg
= 1 - 0.011 = 0.989
If the density of the solution is 1.2 g mL - 1, then the volume of the 100 g solution can be given as:
= 100g / 1.2g mL-1
= 83.33 mL
=83.33 x 10-3 L
∴ Molarity of the solution = 0.056 mol / 83.33 x 10-3 L
= 0.67 M
let the amount of Na2CO3 be x
& that of NaHCO3 be 1-x
Now moles of Na2CO3 = x / 106
& moles of NaHCO3 = 1-x / 84
Now according to question , number of moles of Na2Co3 = number of moles of NaHCO3
Therefore x / 106 = 1-x / 84
84x = 106-106x
84x +106x = 106
190x = 106
Or
x = 106 / 190 = 0.558
Therefore moles of Na2Co3 = 0.558 / 106 = 0.00526
&
moles of NaHCO3 = 1 - 0.558 / 84 = 0.0053
Now Hcl reacts with Na2Co3 & NaHCO3 as follows:
Na2Co3 + 2Hcl 2Nacl + H2o + CO2
NaHCO3 + Hcl Nacl + H2o + CO2
From the above reactions, 1 mol of Na2Co3 will react with 2 mol of Hcl
Therefore 0.00526 mol of Na2Co3 will react with 2 x 0.00526 mol of Hcl & similarly 0.00526 mol of NaHCO3 will react with 0.00526 mol of Hcl
Total moles of Hcl required to react with mixture of of NaHCO3 & Na2Co3
= 2 X 0.00526 + 0.00526 =0.01578 mol
Also according to question 0.1 mol of 0.1 M Hcl is present in 1000 ml
Or
0.01578 mol of 0.1 M Hcl is present in (1000/0.1) x 0.01578 = 157.8 ml
Hence, 158 mL of 0.1 M of HCl is required to react completely with 1 g mixture of Na2CO3 and NaHCO3, containing equimolar amounts of both.
According to question,
300g of 25% solution contains solute = (300 x 25) / 100 = 75g
&
400g of 40% solution contains solute = (400 x 40) / 100 = 160g
Total solute = 75 + 160 = 235g
As given in the question, total solution =300 + 400 = 700g
Percentage of solute in the final solution = (235 x100) / 700 = 33.57%
Percentage of water in the final solution = 100 - 33.5 = 65.43%
Calculation of Molality :
Mass of ethylene glycol = 222.6 (Given)
Molar mass of ethylene glycol [C2H4(OH)2]
= 2 X 12 + 6 x 1 + 2 x 16
= 62
Therefore moles of ethylene glycol
= 222.6g / 62 gmol-1
= 3.59 mol
Mass of water = 200g (Given)
Therefore molality of the solution is = (moles of ethylene glycol / mass of water) x 1000
= (3.59 / 200) x 1000
= 17.95 m
Calculation of Molarity:
Moles of ethylene glycol = 3.59 mol (already calculated)
Total Mass of solution = 200 + 222.6
= 422.6g
Volume of solution = mass / density volume
= 422.6 / 1.072
= 394.22 ml
now molarity of the solution is = (moles of ethylene glycol / volume of solution) x 1000
= (3.59 / 394.22) x 1000
= 9.11 M
1) 15 ppm means : 15 parts per million(106) of the solutions
So, Percent by mass = (mass of chloroform / total mass) x 100
= (15 / 106) x 100
= 1.5 x 10-3 %
2) Molality Mass of chloroform = 15 g
Molar mass of chloroform (CHCl3) = 1 × 12 + 1 × 1 + 3 × 35.5
= 119.5 g mol - 1
Moles of chloroform = 15 / 119.5 = 0.1255 mol
Mass of water = 106
Therefore molality = (moles of chloroform / mass of water ) x 1000
= (0.1255 / 106) x 1000
= 1.255 x 10-4 m
We know that:
Mass percentage of C6H6 

and we also know that:
Mass percentage of CCl4 

Alternatively,
Mass percentage of CCl4= (100 - 15.28)%
= 84.72%
Here, elevation of boiling point ΔTb= (100 + 273) - (99.63 + 273)
= 0.37 K
Mass of water, wl = 500 g
Molar mass of sucrose (C12H22O11),
M2= 11 × 12 + 22 × 1 + 11 × 16 = 342 g mol - 1
Molal elevation constant, Kb= 0.52 K kg mol - 1
We know that:

= (0.37 x 342 x 500) / (0.52 x 1000)
= 121.67 g (approximately)
Hence, 121.67 g of sucrose is to be added.
Mass of acetic acid, w1= 75 g Molar mass of ascorbic acid (C6H8O6),
M2= 6 × 12 + 8 × 1 + 6 × 16
= 176 g mol - 1
Lowering of melting point, ΔTf = 1.5 K
We know that:

= (1.5 x 176 x 75) / (3.9 x 1000)
= 5.08 g (approx)
Hence, 5.08 g of ascorbic acid is needed to be dissolved.
It is given that:
Volume of water, V= 450 mL = 0.45 L
Temperature, T = (37 + 273)K = 310 K
Number of moles of the polymer,
n = 1 / 185000 mol
We know that:
Osmotic pressure, 
= 1/185000 mol X 1/0.45L X 8.314 X 103 Pa L K-1mol-1 X 310K
= 30.98 Pa = 31 Pa (approximately)
Let the total mass of the solution be 100 g and the mass of benzene be 30 g.
∴ Mass of carbon tetrachloride = (100 - 30) g = 70 g
Molar mass of benzene (C6H6) = (6 × 12 + 6 × 1) g mol - 1
= 78 g mol - 1
∴ Number of moles of C6H6 =30/78 mol
= 0.3846 mol
Molar mass of carbon tetrachloride (CCl4) = 1 × 12 + 4 × 35.5
= 154 g mol - 1
∴ Number of moles of CCl4 = 70/154 mol
= 0.4545 mol
Thus, the mole fraction of C6H6 is given as:

= 0.3846 / (0.3846 + 0.4545)
= 0.458
Molarity is given by:
Molarity = moles of solute / Volume of solution in litre
(a) Molar mass of Co(NO3)2.6H2O
= 59 + 2 (14 + 3 × 16) + 6 × 18 = 291 g mol - 1
∴Moles of Co(NO3)2.6H2O = 30 / 291 mol
= 0.103 mol
Therefore, molarity = 0.103 mol / 4.3 L
= 0.024 M
(b) Number of moles present in 1000 mL of 0.5 M H2SO4 = 0.5 mol
∴ Number of moles present in 30 mL of 0.5 M H2SO4 = (0.5 X 30 ) / 1000 mol
= 0.015 mol
Therefore, molarity = 0.015 mol / 0.5 L
= 0.03 M
Molar mass of urea (NH2CONH2) = 2 (1 × 14 + 2 × 1) + 1 × 12 + 1 × 16
= 60 g mol - 1
0.25 molar aqueous solution of urea means:
1000 g of water contains 0.25 mol = (0.25 × 60) g of urea
= 15 g of urea
That is, (1000 + 15) g of solution contains 15 g of urea
Therefore, 2.5 kg (2500 g) of solution contains = (15 X 2500) / (1000+15) g
= 36.95 g
= 37 g of urea (approximately)
Hence, mass of urea required = 37 g
(a) Molar mass of KI = 39 + 127 = 166 g mol - 1
20% (mass/mass) aqueous solution of KI means 20 g of KI is present in 100 g of solution.
That is,
20 g of KI is present in (100 - 20) g of water = 80 g of water
Therefore, molality of the solution = Moles of KI / Mass of water in kg
= 20/166 / 0.08 m
= 1.506 m
= 1.51 m (approximately)
(b) It is given that the density of the solution = 1.202 g mL - 1
∴Volume of 100 g solution = Mass / Density
= 100g / 1.202g mL-1
= 83.19 mL
= 83.19 × 10 - 3 L
Therefore, molarity of the solution = 20/166 mol / 83.19 × 10 - 3 L
= 1.45 M
(c) Moles of KI = 20/166 = 0.12 mol
Moles of water = 80/18 = 4.44 mol
Therefore, mole fraction of KI = Moles of KI / (Moles of KI + Moles of water)
= 0.12 / (0.12+4.44)
= 0.0263
It is given that the solubility of H2S in water at STP is 0.195 m, i.e., 0.195 mol of H2S is dissolved in 1000 g of water.
Moles of water = 1000g / 18g mol-1
= 55.56 mol
∴Mole fraction of H2S, x = Moles of H2S / Moles of H2S + Moles of water
0.195 / (0.195 + 55.56)
= 0.0035
At STP, pressure (p) = 0.987 bar
According to Henry's law:
p= KHx
⇒ KH = p / x
= 0.0987 / 0.0035 bar
= 282 bar
It is given that:
KH = 1.67 × 108Pa
PCO2 = 2.5 atm = 2.5 × 1.01325 × 105Pa
= 2.533125 × 105Pa
According to Henry's law:
PCO2 = KHX
⇒ x = PCO2 / KH
= 2.533125 × 105 / 1.67 × 108
= 0.00152
We can write, 
[Since, is negligible as compared to]
In 500 mL of soda water, the volume of water = 500 mL
[Neglecting the amount of soda present]
We can write:
500 mL of water = 500 g of water
=500 / 18 mole of water
= 27.78 mol of water
Now, nCO2 / nH2O = x
nCO2 / 27.78 = 0.00152
nCO2 = 0.042 mol
Hence, quantity of CO2 in 500 mL of soda water = (0.042 × 44) g
= 1.848 g
It is given that:
PAo = 450 mm of Hg
PBo = 700 mm of Hg
ptotal = 600 mm of Hg
From Raoult's law, we have:
ptotal = PA + PB

Therefore, xB = 1 - xA
= 1 - 0.4
= 0.6
Now, PA = PAo xA
= 450 × 0.4
= 180 mm of Hg
and PB = PBo xB
= 700 × 0.6
= 420 mm of Hg
Now, in the vapour phase: Mole fraction of liquid A = PA / (PA + PB )
=180 / (180+420)
= 180/600
= 0.30
And, mole fraction of liquid B = 1 - 0.30
= 0.70
It is given that vapour pressure of water,PIo = 23.8 mm of Hg
Weight of water taken, w1= 850 g
Weight of urea taken, w2= 50 g
Molecular weight of water, M1= 18 g mol - 1
Molecular weight of urea, M2= 60 g mol - 1
Now, we have to calculate vapour pressure of water in the solution. We take vapour pressure as p1.
Now, from Raoult's law, we have:

Hence, the vapour pressure of water in the given solution is 23.4 mm of Hg and its relative lowering is 0.0173.
Join thousands of students who have improved their academic performance with our comprehensive study resources.