How many geometrical isomers are possible in the following coordination entities?
(i) [Cr(C2O4)3]3-
(ii) [Co(NH3)3Cl3]
(i) For[Cr(C2O4)3]3-, no geometric isomer is possible as it is a bidentate ligand.

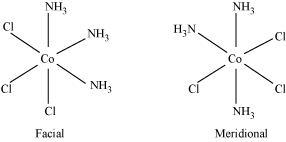
(ii) [Co(NH3)3Cl3]
Two geometrical isomers are possible.
NCERT questions are designed to test your understanding of the concepts and theories discussed in the chapter. Here are some tips to help you answer NCERT questions effectively:
Welcome to the NCERT Solutions for Class 12 Chemistry - Chapter . This page offers a step-by-step solution to the specific question from Excercise 2 , Question 9: How many geometrical isomers are possible in the following coordination entities? (i) [Cr(C2O4)3]....
Comments